Factcheck: Special ‘COVID Vaccine’ Not Made To Experiment On Africans
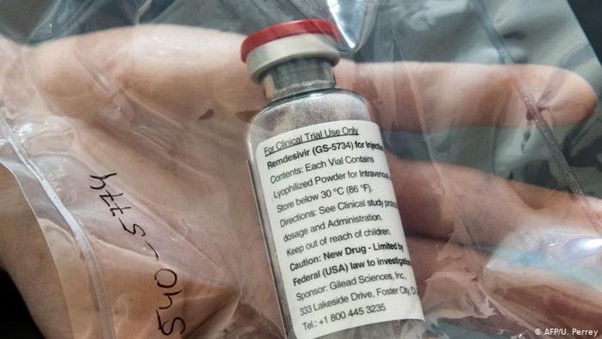
Claim: Remdesivir has been restricted to vaccinate people in African countries in order to experiment on them and “produce genetically modified human beings.”
Verdict: False. Rather, the manufacturer of the drug in the United States gave licences to pharmaceutical companies in other regions, so they could produce generic versions that would be available to developing and underdeveloped countries. The labels on the drugs were merely showing the geographic limitations of the licences.
Full text
A picture has been in circulation in the Nigerian internet space that appears to show that a different kind of remdisivir, “a COVID-19 vaccine, ” was made specially for African countries.
“Not for distribution in U.S., Canada, or EU,” says the labels on the drugs made separately by pharmaceutical companies Hetero Drugs, Cipla, and Jubilant Generics.
In one publication on Nairaland, posted on Jan. 9, the author warned that developed countries were up to something fishy and planned to conduct experiments on Africans using the vaccine. Nairaland is the largest local online forum in Nigeria with over 2.6 million registered members.
“You can see from the pix that Africa has a special vaccine, while Europe is not to be administered. Why is it so? This is more than meets the eyes. The western world is up to somethings that we should not blindly follow,” the forum user, Sunnycliff, wrote.
“Remember it is a nano chip that can be driven by a 5G technology. This is trans-humanism, an experiment of humanity in order to produce genetically modified human beings. Wise up please!”
On Thursday, former federal lawmaker, Dino Melaye, shared pictures of the Cipla drug through his verified page on Facebook. On the side of the pack is stated the instruction that the drug was only meant for use in 47 African countries, including Nigeria.
“What is the meaning of this biko [please]?” he asked in the caption. The post, which was later deleted, had over 700 likes and hundreds of comments. It was shared on different blogs, including Opera News and Sleek Gist.
About Remdisivir
First, remdesivir is not a COVID-19 vaccine. It was originally an investigational drug made by US-based biopharmaceutical company, Gilead Sciences, and considered for the treatment of hepatitis and the Ebola virus infection in 2014.
After the outbreak of the coronavirus, studies found that it proved effective as an antiviral drug in the treatment of COVID-19 patients and shortened recovery period.
In May 2020, the U.S. Food and Drug Administration (FDA) authorised the drug for emergency use “for the treatment of suspected or laboratory-confirmed COVID-19 in adults and children hospitalised with severe disease.” It was the first drug approved by the U.S. regulatory body for COVID-19 treatment.
Shortly afterwards, it was also approved for use by the United Kingdom’s National Health Service (NHS).
The World Health Organisation (WHO), however, stated in November 2020 that it was conditionally advising against the use of the drug as there was no evidence at the time that it improved “survival and other outcomes” in patients.
“The evidence suggested no important effect on mortality, need for mechanical ventilation, time to clinical improvement, and other patient-important outcomes,” the UN agency said after reviewing data from over 7,000 patients across four trials.
Verification
The manufacturers of remdesivir, Gilead, announced last year that they signed “non-exclusive voluntary licensing agreements” with nine pharmaceutical companies based in Egypt, India and Pakistan to expand the drug’s supply.
The companies, namely Cipla, Hetero Labs, and Jubilant Lifesciences, were to ensure the drug’s distribution in 127 countries.
“The countries consist of nearly all low-income and lower-middle-income countries, as well as several upper-middle- and high-income countries that face significant obstacles to healthcare access,” the company explained.
“The licences are royalty-free until the World Health Organization declares the end of the Public Health Emergency of International Concern regarding COVID-19, or until a pharmaceutical product other than remdesivir or a vaccine is approved to treat or prevent COVID-19, whichever is earlier.”
Under the agreements, the companies were exempted from paying royalties to Gilead and could set their own prices for the drug’s generic version they would be producing. “We are confident that these long-standing voluntary licencees can efficiently scale up production of high-quality, low-cost remdesivir,” said Gilead spokesperson, Ryan McKeel.
Generic drugs are generally the same as brand-name and, according to the FDA, have the same “dosage form, safety, strength, route of administration, quality, performance characteristics, and intended use.” What this means is that it is the same remdesivir that is being administered in the various countries.
One of the generic versions of remdesivir, Covifor, has been sold in India since June 2020.
About the label which says the drug is not to be distributed in the U.S., Canada, and European Union countries, the spokesperson for Hetero Labs, one of the licencees, explained that it was “to prevent the drug from being sold illegally on the black market.”
“We think that this photo is part of a malicious campaign to create confusion around this drug,” the spokesperson added about the claim in circulation. Gilead had confirmed this too, telling Reuters that the labels only indicated restrictions in where the licencees could distribute the generic drugs.
Conclusion
The claims are false. Remdesivir is not a vaccine specially made for Africans but an antiviral drug found helpful in treating COVID-19 patients and whose generic versions are being produced for countries in other regions of the world, including Africa. The generic drug has exactly the same qualities as the brand-name drug.
The researcher produced this fact-check per the Dubawa 2020 Fellowship partnership with HumAngle to facilitate the ethos of “truth” in journalism and enhance media literacy in the country.
Support Our Journalism
There are millions of ordinary people affected by conflict in Africa whose stories are missing in the mainstream media. HumAngle is determined to tell those challenging and under-reported stories, hoping that the people impacted by these conflicts will find the safety and security they deserve.
To ensure that we continue to provide public service coverage, we have a small favour to ask you. We want you to be part of our journalistic endeavour by contributing a token to us.
Your donation will further promote a robust, free, and independent media.
Donate HereStay Closer To The Stories That Matter





